Abstract
Introduction and objectives: Myelofibrosis (MF) has the worst outcome amongst various myeloproliferative neoplasms. Its prognosis is determined by clinicopathologic features and mutations in key driver genes. An increasing number of gene mutations involving various biological pathways in myeloid malignancies has been discovered. The prognostic significance of these mutations have not been clearly defined. In this study, we aim to describe the genomic characteristic in a large cohort of MF patients and identify clinical and molecular predictors of outcome.
Methods: We evaluated the genetic profile of 101 patients with MF (primary, N=70; secondary, N=30) using next-generation sequencing with a 54-gene panel comprising: ABL1, ASXL1, ATRX, BCOR, BCORL1, BRAF, CALR, CBL, CBLB, CBLC, CDKN2A, CEBPA, CSF3R, CUX1, DNMT3A, ETV6, EZH2, FBXW7, FLT3, GATA1, GATA2, GNAS, HRAS, IDH1, IDH2, IKZF1, JAK2, JAK3, KDM6A, KIT, KMT2A, KRAS, MPL, MYD88, NOTCH1, NPM1, NRAS, PDGFRA, PHF6, PTEN, PTPN11, RAD21, RUNX1, SETBP1, SF3B1, SMC1A, SMC3, SRSF2, STAG2, TET2, TP53, U2AF1, WT1, ZRSR2. Multivariate cox regression analysis was used to determine prognostic factors for overall survival (OS) and leukemia-free survival (LFS).
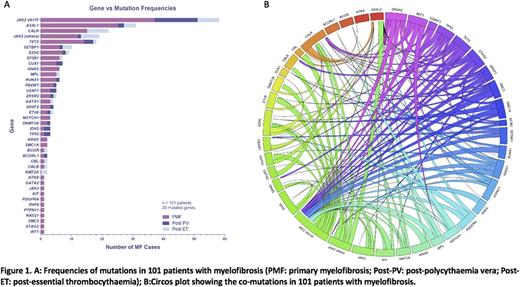
Results: We identified mutations in 39 genes implicated in myeloid malignancies (Figure 1A). 96 patients (95%) with MF had a mutation in 1 or more genes: 14 patients (13.9%) had 1 mutation, 38 patients (37.6%) had 2 mutations, 18 patients (17.8%) had 3 mutations, 15 patients (14.9%) had 4 mutations, 7 patients (6.9%) had 5 mutations and 4 patients (4%) had 6 or more mutations. TET2 / JAK2V617F (16 patients, 15.9%), ASXL1 / JAK2V617F (12 patients, 11.9%) and ASXL1/CALR (10 patients, 9.9%) were the most frequently co-mutated genes (Figure 1B). Other JAK2 variants occurred concomitantly with JAK2V617F in 10 patients (9.9%) and CALR mutations in 4 patients (4%) mutations. Other frequently concomitant mutations included CUX1 / JAK2V617F (6 patients, 5.9%), EZH2 / JAK2V617F (6 patients, 5.9%), RUNX1 / JAK2V617F (5 patients, 5%), SF3B1 / JAK2V617F (5 patients, 5%), SETBP1 / JAK2V617F (4 patients, 4%) and ZRSR2 / JAK2V617F (4 patients, 4%).
The median follow-up of the cohort was 49 (1-256) months. The 5-year and 10-year OS were 66.3% and 35.4%. The 5-year and 10-year LFS of were 84% and 63.3%. There were no statistically significant differences in OS and LFS between primary and secondary MF. Significant negative prognostic indicators were identified on multivariate analysis, including male gender (P=0.044), age > 65 years (P=0.044), Hb < 10g/dL (P=0.001), mutated CUX1 (P=0.003) and mutated TP53 (P=0.043) for OS, and Hb < 10g/dL (P=0.007), mutated TP53 (P=0.043) and mutated IDH2 (P=0.001) for LFS. In primary MF, inferior prognostic indicators included male gender (P=0.031), Hb < 10g/dL (P=0.002), platelet count < 100 x 109/L (P=0.021), mutated TET2 (P=0.011) and mutated CUX1 (P=0.011) for OS; and Hb < 10g/dL (P=0.027), mutated RUNX1 (P=0.019) and mutated DNMT3A (P=0.004) for LFS. In JAK2V617F positive MF, inferior prognostic indicators included mutated ASXL1 (P=0.006) and mutated SRSF2 (P<0.001) for OS; and mutated U2AF1 (P=0.037) for LFS.
Conclusion: Our study demonstrated unique molecular profiles and prognostic predictors of outcome in Chinese patients with MF.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.